What is the pressure (in atmospheres)of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 735 mmHg and h = 8.3 cm? 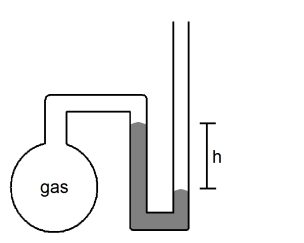
Correct Answer:
Verified
Q93: What is the pressure (mmHg)of the sample
Q94: Calculate the molar mass of a gaseous
Q95: Calculate the density of SO2 gas, in
Q96: An aerosol can with a volume of
Q97: How many grams of N2O, nitrous oxide,
Q99: A block of dry ice (solid CO2,
Q100: What is the pressure (mmHg)of the sample
Q101: A particular coal sample contains 2.32% S.When
Q102: Today is a beautiful day for a
Q103: The following data describes an initial and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents