Calculate the Energy Change for the Reaction K(g)+ Br(g) K+(g)+ Br- (G)
Given the Following Ionization Energy (IE)and Electron
Calculate the energy change for the reaction K(g) + Br(g) K+(g) + Br- (g)
Given the following ionization energy (IE) and electron affinity (EA) values 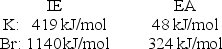
A) -1,092 kJ/mol
B) -95 kJ/mol
C) 95 kJ/mol
D) 1,092 kJ/mol
E) 1,187 kJ/mol
Correct Answer:
Verified
Q4: Complete this statement: Coulomb's law states
Q6: Which of the following solids would have
Q15: The Lewis dot symbol for the S
Q16: Which one of the following is most
Q16: The Lewis dot symbol for the calcium
Q22: Which of the bonds below would have
Q26: The covalent bond with the greatest polarity
Q27: Arrange the elements Ba, Br, and Ga
Q29: A polar covalent bond would form in
Q34: Which one of these polar covalent bonds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents