How much energy (heat) is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 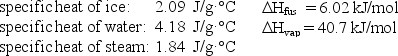
A) 40.2 kJ
B) 157.8 kJ
C) 1,086 kJ
D) 2,570 kJ
E) 22,957 kJ
Correct Answer:
Verified
Q63: A liquid boils when its
A) vapor pressure
Q63: Use the graph of vapor pressure to
Q66: Which of the following constants is/are
Q67: Use the graph of vapor pressure to
Q69: How much energy (heat)is required to convert
Q69: Which of the following phase changes is
Q70: A phase change from the gas phase
Q70: Which of the following constants is/are
Q72: The atomic planes in a graphite crystal
Q73: The vapor pressure of a liquid in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents