At 700 K, the rate constant for the following reaction is 6.2 × 10-4 min-1. 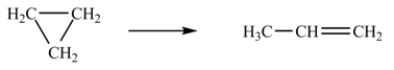 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene?
A) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 × 10-4 min
E) 280 min
Correct Answer:
Verified
Q24: A reaction was experimentally determined to follow
Q26: The isomerization of cyclopropane to form propene
Q27: Ammonium ion (NH4+)reacts with nitrite ion (NO2-)to
Q30: A certain reaction, reaction A
Q32: The data below were determined for
Q34: Benzoyl chloride, C6H5COCl, reacts with water to
Q34: At 25°C the rate constant for the
Q36: A city's water supply is contaminated with
Q36: The following initial rate data apply
Q38: The first-order decomposition, A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents