For the chemical reaction system described by the diagram below, which statement is true? 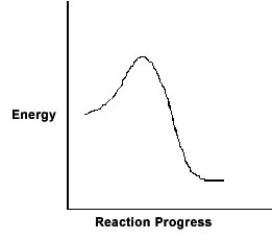 If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction?
If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction?
A) 120 kJ/mol
B) 70 kJ/mol
C) 95 kJ/mol
D) 25 kJ/mol
E) -70 kJ/mol
Correct Answer:
Verified
Q68: At 25°C, by what factor is the
Q69: What is the slope of an
Q69: When the concentrations of reactant molecules are
Q70: The activation energy for the following
Q70: The Arrhenius equation is k = Ae-Ea/RT.
Q72: The activation energy for the reaction
Q73: The activation energy for the reaction
Q74: The following mechanism has been suggested
Q75: The isomerization of cyclopropane follows first order
Q78: If Ea for a certain biological reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents