The gas phase reaction of nitrogen dioxide and carbon monoxide was found experimentally to be second-order with respect to NO2, and zero-order with respect to CO below 25°C. NO2 + CO NO + CO2
Which one of the following mechanisms is consistent with the observed reaction order?
A) NO2 + 2CO 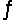 N + 2CO2 fast N + NO2 2NO slow
N + 2CO2 fast N + NO2 2NO slow
B) NO2 + 2CO N + 2CO2 slow N + NO2 2NO fast
C) NO2 + NO2 NO3 + NO fast NO3 + CO NO2 + CO2 slow
D) NO2 + NO2 NO3 + NO slow NO3 + CO NO2 + CO2 fast
Correct Answer:
Verified
Q83: Complete the following statement: A catalyst
A) increases
Q85: The following mechanism has been suggested
Q86: The first-order decomposition of phosphene to
Q87: The following reaction in aqueous solution
Q89: The rate law for the reaction
Q92: The activation energy of a certain uncatalyzed
Q94: Aspirin, C9H8O4, slowly decomposes at room
Q95: With respect to the figure below, which
Q97: Which of the following statements is false
A)
Q98: For the reaction X2 + Y
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents