Short Answer
Use the table of data shown below to calculate the average rate of the reaction A B between 10 s and 20 s. 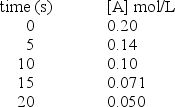
Correct Answer:
Verified
Related Questions
Q115: For the first-order reaction 2N2O5
Q116: The reaction 2A
Q117: Nitrogen pentoxide decomposes by a first-order
Q118: Nitric acid is formed by the
Q119: Nitric acid is formed by the
Q121: The rate determining step must be the
Q123: The rate constant of a first-order
Q124: B is a catalyst in the following
Q126: It is possible for the following
Q130: It is possible for the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents