Given the following information: 2A(g) + B(g) 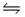
A2B(g) Kp1
2A(g) + C2(g) 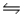
2AC(g) Kp2
3/2 A2 + B(g) + C(g) 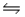
AC(g) + A2B(g) Kp3
Which relationship represents the equilibrium constant for the reaction:
4A(g) + C2(g) + A2B(g) 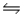
3A2(g) + B(g) + 2C(g)
A) Knet = Kp1 x Kp2 x Kp3
B) Knet = Kp1 x Kp2 x 2 Kp3
C) Knet = Kp1 x Kp2 x Kp32
D) Knet = Kp1 x Kp2/ Kp3
E) Knet = Kp1 x Kp2/ Kp32
Correct Answer:
Verified
Q1: The equilibrium constant expression for the reaction
Q2: The equilibrium constant expression for the reaction:
Q3: The equilibrium constant for the reaction Ni(s)+
Q5: The solubility of silver bromide can be
Q7: The solubility of silver chloride can be
Q9: Calculate Kc for the reaction 2HI(g)
Q10: Consider the two gaseous equilibria: 
Q11: A reaction with an equilibrium constant Kc
Q11: The brown gas NO2 and the colorless
Q15: A reaction with an equilibrium constant Kc
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents