Hydrogen iodide decomposes according to the equation 2HI(g) 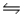 H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of HI at equilibrium.
H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of HI at equilibrium.
A) 0.138 M
B) 0.220 M
C) 0.550 M
D) 0.275 M
E) 0.0275 M
Correct Answer:
Verified
Q34: At 250ºC, the equilibrium constant Kp for
Q35: Consider the reaction N2(g)+ O2(g) 
Q36: Consider the following reactions and their associated
Q37: At 700 K, the reaction 2SO2(g)+ O2(g)
Q38: Sodium carbonate, Na2CO3(s), can be prepared by
Q40: Consider the following equilibria: 2SO3(g) 
Q41: For the following reaction at equilibrium,
Q42: Consider this reaction at equilibrium at a
Q43: For the following reaction at equilibrium
Q44: The reaction 2SO3(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents