At 340 K, Kp = 69 for the reaction H2(g) + I2(g) 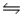
2HI(g) .50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K.What is the total pressure inside the cylinder when the system comes to equilibrium?
A) 2.60 atm
B) 1.76 atm
C) 0.424 atm
D) 2.18 atm
E) 10.9 atm
Correct Answer:
Verified
Q22: For the reaction SO2(g)+ NO2(g) 
Q23: Kp for the reaction of SO2(g)with O2
Q24: Kp for the reaction CO2(g)+ C(s)
Q25: For the nitrogen fixation reaction 3H2(g)+ N2(g)
Q26: At 400ºC, Kc = 64 for the
Q28: Hydrogen iodide decomposes according to the equation
Q29: Kp for the reaction 4CuO(s) 
Q30: At 35ºC, the equilibrium constant for the
Q31: At 700 K, the reaction 2SO2(g)+ O2(g)
Q32: Phosgene, COCl2, a poisonous gas, decomposes according
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents