Hydrogen iodide decomposes according to the equation:
2HI(g) 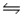
H2(g)+ I2(g), Kc = 0.0156 at 400ºC
A 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of H2 equilibrium.
Correct Answer:
Verified
Q89: Calcium carbonate decomposes at high temperatures to
Q90: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q91: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q92: The data below refer to the following
Q93: Consider the equilibrium C(s)+ H2O(g)
Q95: Consider the chemical reaction 2NH3(g) 
Q96: Consider the equilibrium equation C(s)+ H2O(g)
Q97: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q98: The Kc for the reaction CO2(g)+ H2(g)
Q99: Hydrogen iodide decomposes according to the equation:
2HI(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents