Consider the following equilibrium,
4NH3(g)+ 3O2(g) 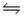
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant after ammonia was added to the system.
Correct Answer:
Verified
Q92: The data below refer to the following
Q93: Consider the equilibrium C(s)+ H2O(g)
Q94: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q95: Consider the chemical reaction 2NH3(g) 
Q96: Consider the equilibrium equation C(s)+ H2O(g)
Q98: The Kc for the reaction CO2(g)+ H2(g)
Q99: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q100: Consider the equilibrium C(s)+ H2O(g)
Q101: When the reaction 2O3(g) Q102: The equilibrium between carbon dioxide gas and![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents