In the reaction: CH3COOH(aq) + NH2- (aq) 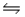 CH3COO- (aq) + NH3(aq) , the conjugate acid-base pairs are:
CH3COO- (aq) + NH3(aq) , the conjugate acid-base pairs are:
A) pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3
B) pair 1: CH3COOH and NH3; pair 2: NH2- and CH3COO-
C) pair 1: CH3COOH and NH2- ; pair 2: NH3 and CH3OO-
D) pair 1: CH3COOH and CH3COO- ; pair 2: NH4+ and NH3
E) pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3+
Correct Answer:
Verified
Q2: Which of the following is not a
Q3: The hydronium ion and the hydroxide ion,
Q4: Identify the conjugate base of HSO4-.
A) OH-
B)
Q9: In the reaction HSO4-(aq)+ OH-(aq) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents