Predict the direction in which the equilibrium will lie for the reaction C6H5COO- + HF 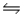
C6H5COOH + F- .
Ka(C6H5COOH) = 6.5 × 10-5; Ka(HF) = 7.1 × 10-4
A) to the right
B) to the left
C) in the middle
Correct Answer:
Verified
Q83: The equilibrium expression for the hydrolysis of
Q89: Which of these species will act as
Q91: Predict the direction in which the equilibrium
Q92: Calculate the concentration of malonate ion (C3H2O42-)in
Q93: Predict the direction in which the equilibrium
Q94: Hydrosulfuric acid is a diprotic acid, for
Q95: Which of the following yields an acidic
Q95: The oxides SO2 and N2O5 will form
Q100: Predict the direction in which the equilibrium
Q106: Which one of these salts will form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents