Consider the weak bases below and their Kb values: 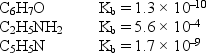 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
A) C5H5NH+ < C6H7OH < C2H5NH
B) C6H7OH < C5H5NH+ < C2H5NH
C) C5H5NH+ < C2H5NH3+ < C6H7OH
D) C6H7OH < C2H5NH3+< C5H5NH+
E) C2H5NH3+< C5H5NH+ < C6H7OH
Correct Answer:
Verified
Q105: The pH of a 0.095 M solution
Q107: Which one of these salts will form
Q109: Calculate the pH of a 0.021 M
Q109: Which one of these salts will form
Q110: For H3PO4, Ka1 = 7.3 × 10-3,
Q112: What is the pH of a 0.20
Q116: Calculate the pH of a 0.20 M
Q117: What is the pH of a
Q118: What is the pH of a 0.023
Q120: What is the pH of a 0.15
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents