Acid dissociation constants for phosphoric acid are given below. 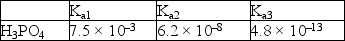 A buffer with a pH = 7.4 can best be made by using
A buffer with a pH = 7.4 can best be made by using
A) H3PO4 and NaH2PO4.
B) NaH2PO4 and Na2HPO4.
C) Na2HPO4 and Na3PO4.
D) only NaH2PO4.
E) only Na2HPO4.
Correct Answer:
Verified
Q3: Consider a buffer solution prepared from
Q4: Which of the following yields a buffer
Q15: Which of the following is the most
Q17: Which of the following yields a buffer
Q17: Calculate the pH of a buffer solution
Q22: What mass of sodium fluoride must be
Q24: Calculate the pH at the equivalence point
Q26: What mass of ammonium nitrate must be
Q37: In which one of the following solutions
Q55: Calculate the pH of the solution resulting
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents