Calculate the equilibrium constant Kc for the following overall reaction:
AgCl(s)+ 2CN-(aq) 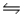
Ag(CN)2-(aq)+ Cl-(aq)
For AgCl, Ksp = 1.6 × 10-10; for Ag(CN)2-, Kf = 1.0 × 1021.
Correct Answer:
Verified
Q104: Will Fe(OH)3 precipitate from a buffer solution
Q105: Solid sodium iodide is slowly added to
Q106: Ammonium chloride solutions are slightly acidic, so
Q107: An environmental chemist obtained a 200.mL sample
Q108: Solid sodium iodide is slowly added to
Q110: Calculate the molar solubility of lead (II)fluoride
Q111: A sample of rainwater collected near a
Q112: An environmental chemist obtained a 200.mL sample
Q113: An environmental chemist obtained a 200.mL sample
Q114: Bromothymol blue is a common acid-base indicator.It
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents