Sulfur can be separated from lead in the mineral galena, PbS(s) , by "roasting" the ore in the presence of oxygen as shown in the following reaction:
2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g)
Calculate S° for this reaction using the thermodynamic data provided below. 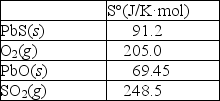
A) -410 J/K·mol
B) -161.5 J/K·mol
C) -47.7 J/K·mol
D) 21.8 J/K·mol
E) 43.5 J/K·mol
Correct Answer:
Verified
Q7: Which response includes all the following
Q12: Which of these species would you expect
Q13: Which one of the following reactions
Q14: Which response includes all of the following
Q15: Determine
Q16: Arrange these compounds in order of increasing
Q18: Which of these species would you expect
Q19: Which of the following processes would
Q22: Ozone (O3)in the atmosphere can reaction
Q31: Which of the following is consistent
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents