In the gas phase, methyl isocyanate (CH3NC) isomerizes to acetonitrile (CH3CN) , H3C-N C (g) 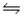
H3C-C N (g)
With H° = -89.5 kJ/mol and G° = - 73.8 kJ/mol at 25°C.Find the equilibrium constant for this reaction at 100°C.
A) 1.68 × 10-10
B) 5.96 × 109
C) 2.16 × 1010
D) 4.63 × 10-11
E) 8.64 × 1012
Correct Answer:
Verified
Q72: For the reaction SbCl5(g) 
Q73: Find the temperature at which Kp
Q74: Find the temperature at which Kp
Q75: A sample of solid naphthalene is introduced
Q76: For the reaction CuS(s)+ H2(g)
Q78: For the reaction SbCl5(g) 
Q79: Rubidium has a heat of vaporization
Q80: For the reaction CuS(s)+ H2(g)
Q81: For the reaction 3H2(g)+ N2(g) 
Q82: Is the reaction SiO2(s)+ Pb(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents