For the reaction 3H2(g)+ N2(g) 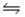
2NH3(g), Kc = 9.0 at 350°C.In what direction does the reaction proceed when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
Correct Answer:
Verified
Q82: Is the reaction SiO2(s)+ Pb(s)
Q83: Under what conditions (always, never, high temperature
Q84: Under which of the following conditions
Q85: Calculate the free energy of formation
Q86: What is the free energy change
Q88: For the reaction 3H2(g)+ N2(g)
Q89: Under what conditions (always, never, high
Q90: Predict the signs (-, +, or
Q91: For the reaction 3H2(g)+ N2(g)
Q92: Predict the signs (-, +, or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents