Consider the following standard reduction potentials in acid solution: 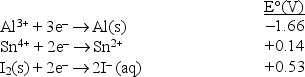 Which is the weakest oxidizing agent in this list?
Which is the weakest oxidizing agent in this list?
A) Al3+(aq)
B) Al(s)
C) I-(aq)
D) I2(s)
E) Sn4+(aq)
Correct Answer:
Verified
Q25: A galvanic cell has the overall
Q36: Consider the following electrochemical cell: U |
Q40: Calculate E°cell for a silver-aluminum cell
Q41: Given the following standard reduction potentials,
Q42: Calculate
Q44: Consider the following standard reduction potentials in
Q46: Which one of the following reagents is
Q47: Consider the following standard reduction potentials in
Q48: The half-cell reaction for the oxidation
Q57: Which one of the following reagents is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents