Write the formula of the weakest reducing agent.given the following standard reduction potentials in acid solution 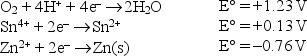
Correct Answer:
Verified
Q116: Which of these metals will not reduce
Q121: Determine the equilibrium constant for the following
Q122: Calculate
Q123: When a solution of a certain gadolinium
Q124: Aluminum metal is formed by the electrolysis
Q126: Calculate E°cell for the following electrochemical cell:
Ni(s)|
Q127: Aluminum metal is formed by the electrolysis
Q128: Gold can be electrochemically "plated" onto
Q129: Will H2(g)form when Sn is placed in
Q130: If the cell emf of a Zn-Cu
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents