Write the formula of the strongest reducing agent.given the following standard reduction potentials in acid solution 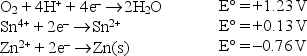
Correct Answer:
Verified
Q131: What current is needed to deposit 0.500
Q132: Write the formula of the strongest oxidizing
Q133: Calculate E°cell for the following electrochemical cell:
Mn(s)|
Q134: Will H2(g)form when Ag is placed in
Q135: Calculate
Q137: Determine the equilibrium constant for the following
Q138: Write a balanced equation for a spontaneous
Q139: Will H2(g)form when Fe is placed in
Q140: Will H2(g)form when Cu is placed in
Q141: How many moles of silver metal are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents