Consider the following decay series: 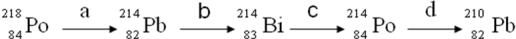 Which type of nuclear process occurs in steps c and d, respectively?
Which type of nuclear process occurs in steps c and d, respectively?
A) ( emission, emission)
B) ( emission, emission)
C) ( emission, positron emission)
D) ( emission, emission)
E) (positron emission, emission)
Correct Answer:
Verified
Q9: Which of the following represents a rule
Q12: What is the missing symbol in
Q12: Beta particles are identical to
A) protons.
B) helium
Q13: Predict the other product of the
Q14: As a result of alpha emission, the
Q15: When atoms of beryllium-9 are bombarded with
Q18: Radium-226 decays by alpha emission.What is its
Q19: Sulfur-35 decays by beta emission.The decay product
Q21: Consider the following decay series: 
Q22: Consider the following decay series:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents