Consider the following decay series: 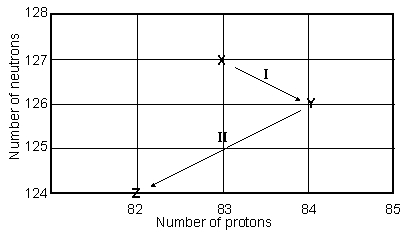 What type of nuclear process occurs at the transformation labeled II?
What type of nuclear process occurs at the transformation labeled II?
A) ( emission)
B) ( emission)
C) positron emission
D) electron capture
E) gamma radiation
Correct Answer:
Verified
Q35: The only stable isotope of iodine is
Q36: Find the nuclear binding energy of potassium-40
Q37: A typical radius of an atomic nucleus
Q38: The only stable isotope of aluminum is
Q39: Consider the following decay series: 
Q43: The half-life of 90Sr is 29 years.
Q45: Cobalt-60 is a beta emitter with a
Q53: How old is a bottle of wine
Q54: Cobalt-60 is a beta emitter with a
Q60: Polonium-208 is an alpha emitter with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents