For the reaction 3Fe(s) + C(s) 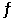 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C.Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
A) 1.0°C
B) 970°C
C) 700°C
D) 1000°C
E) 760°C
Correct Answer:
Verified
Q47: What is the total number of
Q52: Write a balanced chemical equation illustrating roasting.
Q53: Write the chemical formula of corundum.
Q55: Write a balanced chemical equation illustrating the
Q55: Three means used to concentrate ores are
Q56: Potassium superoxide, KO2(s), is used in the
Q59: How much energy (kJ)is released if
Q60: Write the chemical formula of pyrite.
Q61: Cast iron as it is prepared in
Q62: Bauxite (Al2O3·2H2O)ore is the principal commercial source
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents