The equilibrium 3Fe(s) + C(s) 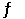 Fe3C(s) is established in a solid solution.For such a solution, one can write an equilibrium constant expression in the usual way except that the concentrations that refer to solids in the solid solution are included.Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
Fe3C(s) is established in a solid solution.For such a solution, one can write an equilibrium constant expression in the usual way except that the concentrations that refer to solids in the solid solution are included.Determine the equilibrium constant for the formation of cementite from iron and carbon at 680°C.(Given: for this reaction at 25°C, H° = 21 kJ/mol and S° = 20.4 J/mol·K)
A) 0.75
B) 0.33
C) 3.1
D) 0.82
E) 1.2
Correct Answer:
Verified
Q23: Which of the following is not a
Q37: Argentite, chalcocite and cinnabar are all examples
Q39: The Hall process involves the reduction of
Q41: Write the chemical formula of epsomite (sold
Q42: The following reaction is used to
Q44: The following reaction is used to
Q45: Write the chemical formula of dolomite that
Q46: Why is cryolite, Na3AlF6, mixed with alumina
Q47: How much energy (kJ)is released when
Q48: Write two balanced chemical equations for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents