The coordination compound shown below has 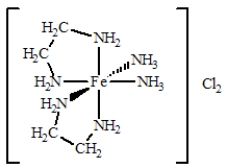
A) two monodentate ligands and two bidentate ligands.
B) three monodentate ligands and two bidentate ligands.
C) four monodentate ligands and two bidentate ligands.
D) one monodentate ligand and four bidentate ligands.
E) four bidentate ligands.
Correct Answer:
Verified
Q21: Which of these square planar complex ions
Q23: Which response gives the correct coordination number
Q24: Which of these octahedral complexes can have
Q34: The correct formula for pentaamminechloroplatinum(IV) chloride is:
A)
Q35: The correct name for K3[Fe(C2O4)3] is:
A) tripotassium
Q37: In the complex ion [Cr(C2O4)2(H2O)2]-, the oxidation
Q40: The correct name for [Fe(CO)6]Br3 is:
A) hexacarbonyliron(II)
Q41: Which one of these complex ions would
Q55: Which of the following complexes has optical
Q58: How many unpaired electrons are there in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents