The best name for the complex shown below is 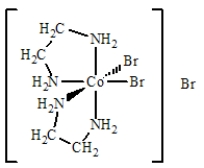
A) cobalt(III) bis(ethylenediamine) bromide.
B) dibromobis(ethylenediamine) cobalt(III) bromide.
C) dibromidedi(ethylenediamine) cobalt(III) bromide.
D) dibromodiethylenediaaminecobalt(III) bromide.
E) tribromobis(ethylenediamine) cobalt(III) .
Correct Answer:
Verified
Q5: The total number of electrons in the
Q15: In K4[Fe(CN)6], how many 3d electrons does
Q25: The correct formula for dicarbonylsilver (I)ion is:
A)Ag(CO)2
B)[Ag(CO)2]+
C)[Ag(CO)2]2+
D)[Ag(CO)2]-
E)[Ag(CO)2]2-
Q28: In the complex ion [Co(en)2Br2]+, the oxidation
Q29: Give the coordination number (C.N.) and oxidation
Q30: The correct name for Fe(CO)5 is:
A) pentacarbonyliron(V)
B)
Q31: In the coordination compound [Pt(NH3)2Cl2], the coordination
Q33: In the coordination compound K2[Co(en)Cl4], the coordination
Q38: The correct formula for sodium tetracyanonickelate(II) ion
Q39: In the coordination compound [Cr(NH3)(en)2Cl]Br2, the coordination
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents