Which of these electron energy level patterns would absorb light with the shortest wavelength? 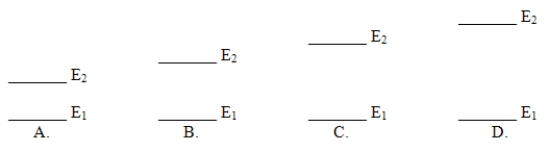
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Q35: The correct name for K3[Fe(C2O4)3] is:
A) tripotassium
Q41: Which one of these complex ions would
Q46: How many unpaired electrons does the manganese
Q48: Which of these complex ions would absorb
Q48: Two well-known complex ions containing Ni
Q51: In the complex ion [ML6]n+, Mn+ has
Q53: Which of the following complexes has optical
Q55: Which of the following complexes has optical
Q58: How many unpaired electrons are there in
Q59: In the complex ion [ML6]n+, Mn+ has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents