What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 736 mmHg and h = 9.2 cm? 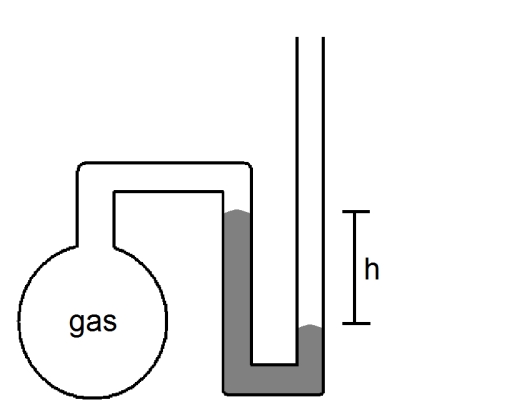
A) 92 mmHg
B) 644 mmHg
C) 736 mmHg
D) 828 mmHg
Correct Answer:
Verified
Q14: A small bubble rises from the bottom
Q15: A sample of a gas occupies 1.40
Q16: What will happen to the height (h)of
Q16: A pressure that will support a column
Q18: Calculate the number of moles of gas
Q21: Determine the molar mass of chloroform gas
Q23: Calculate the density of Br2(g)at 59.0°C and
Q24: Two moles of chlorine gas at 20.0°C
Q35: Calculate the mass, in grams, of 2.74
Q38: Calculate the volume occupied by 35.2 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents