Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l) , using the following information: 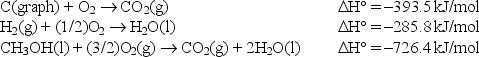
A) -1,691.5 kJ/mol
B) -238.7 kJ/mol
C) 1691.5 kJ/mol
D) 47.1 kJ/mol
E) -47.1 kJ/mol
Correct Answer:
Verified
Q27: Octane (C8H18)undergoes combustion according to the
Q28: To which one of the following
Q29: Calculate the standard enthalpy change for
Q30: Pentaborane B5H9(s)burns vigorously in O2 to
Q31: Given the thermochemical equation 2SO2 +
Q32: Find the standard enthalpy of formation
Q35: Styrene, C8H8, is one of the
Q36: A 0.1326 g sample of magnesium
Q37: For the reaction Q38: Glycine, C2H5O2N, is important for biological![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents