Multiple Choice
During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g)
Calculate the standard enthalpy change for the above reaction given: 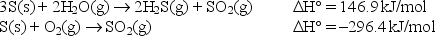
A) -1036.1 kJ/mol
B) -742.3 kJ/mol
C) -149.5 kJ/mol
D) 443.3 kJ/mol
E) 742.3 kJ/mol
Correct Answer:
Verified
Related Questions
Q27: Octane (C8H18)undergoes combustion according to the
Q35: Styrene, C8H8, is one of the
Q36: A 0.1326 g sample of magnesium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents