At 25°C, the following heats of reaction are known: 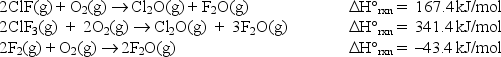
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
A) -217.5 kJ/mol
B) -130.2 kJ/mol
C) 217.5 kJ/mol
D) -108.7 kJ/mol
E) 465.4 kJ/mol
Correct Answer:
Verified
Q65: Which of the following processes always results
Q75: The enthalpy change when a strong acid
Q77: Calculate the amount of work done
Q77: Given the following
Q82: The heat of solution of calcium chloride
Q83: The heat of solution of NH4NO3 is
Q85: What is the standard enthalpy of formation
Q95: Define specific heat.
Q97: A 0.1946 g piece of magnesium metal
Q104: Find
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents