Use the Born-Haber cycle to calculate the lattice energy of KCl(s) given the following data: H(sublimation) K = 79.2 kJ/mol
I1 (K) = 418.7 kJ/mol
Bond energy (Cl-Cl) = 242.8 kJ/mol
EA (Cl) = 348 kJ/mol
H 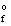 (KCl(s) ) = -435.7 kJ/mol
(KCl(s) ) = -435.7 kJ/mol
A) -165 kJ/mol
B) 288 kJ/mol
C) 629 kJ/mol
D) 707 kJ/mol
E) 828 kJ/mol
Correct Answer:
Verified
Q23: Use the Born-Haber cycle to calculate
Q24: Which of the elements listed below has
Q25: Which of the following covalent bonds is
Q26: A polar covalent bond would form in
Q27: Which one of these polar covalent bonds
Q29: The bond in which one of the
Q30: Which of the elements listed below is
Q31: Which of the bonds below would have
Q32: A nonpolar covalent bond (i.e., pure covalent)would
Q33: Classify the O - H bond in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents