Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever.What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 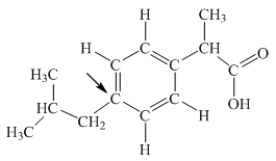
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer:
Verified
Q41: Which one of the following molecules is
Q46: The F -Cl -F bond angles in
Q48: Which one of the following molecules is
Q52: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q55: Predict the geometry and polarity of the
Q55: Complete this sentence: The PCl5 molecule has
A)nonpolar
Q58: Which of the following species has the
Q58: Ibuprofen is used as an analgesic for
Q59: Which one of the following molecules has
Q71: Indicate the type of hybrid orbitals used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents