Use the following data to determine the molar heat of vaporization of chlorine. 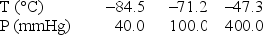
A) 34,700 J
B) 21,900 J
C) 317 J
D) 712 J
E) 9.99 kJ
Correct Answer:
Verified
Q64: The vapor pressure of ethanol is 400
Q65: Which one of the following elements would
Q67: The molar enthalpy of vaporization of carbon
Q70: Solid iodine has a vapor pressure of
Q71: Calculate the amount of heat needed
Q72: Find the temperature at which ethanol boils
Q73: Which of the following gases would have
Q74: Find the temperature at which water boils
Q81: Octane is a liquid component of gasoline.Given
Q82: The molar enthalpy of vaporization of boron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents