Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 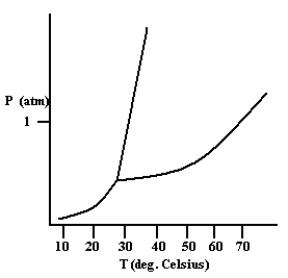
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer:
Verified
Q71: Calculate the amount of heat needed
Q72: Find the temperature at which ethanol boils
Q73: Which of the following gases would have
Q73: The vapor pressure of a liquid in
Q74: Find the temperature at which water boils
Q77: The normal boiling point of bromine is
Q78: The molar enthalpy of vaporization of hexane
Q80: Calculate the amount of heat that
Q87: The molar heats of sublimation and fusion
Q106: Indicate all the types of intermolecular forces
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents