The isomerization of cyclopropane to form propene is a first-order reaction. 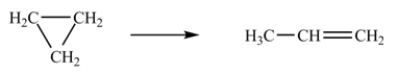 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min.What is the half-life of cyclopropane at 760 K?
A) 3.4 10-2 min
B) 2.5 min
C) 23 min
D) 29 min
E) 230 min
Correct Answer:
Verified
Q32: Appropriate units for a second-order rate constant
Q33: A certain reaction A products
Q34: A certain reaction A products
Q34: Benzoyl chloride, C6H5COCl, reacts with water to
Q36: A certain first-order reaction A
Q39: A certain first-order reaction A
Q39: The isomerization of cyclopropane to propene follows
Q43: For what order reaction does the half-life
Q56: The reaction 2NO2(g)
Q72: The activation energy for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents