For the chemical reaction system described by the diagram below, which statement is true? 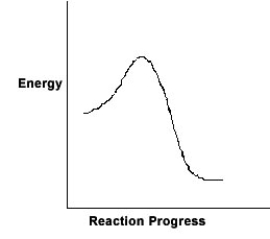
A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.
Correct Answer:
Verified
Q69: When the concentrations of reactant molecules are
Q72: Given that Ea for a certain biological
Q75: The following reaction in aqueous solution was
Q76: Solids cannot react with gases.
A)1 and 2
B)1
Q78: The rate law for the reaction 2NO2
Q80: With respect to the figure below, which
Q81: Use the table of data shown below
Q82: At a certain temperature, the data below
Q92: The activation energy of a certain uncatalyzed
Q98: Nitrous oxide (N2O)decomposes at 600°C according
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents