For the chemical reaction system described by the diagram below, which statement is true? 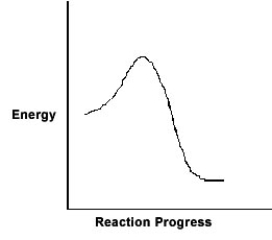 If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction?
If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction?
A) 120 kJ/mol
B) 70 kJ/mol
C) 95 kJ/mol
D) 25 kJ/mol
E) -70 kJ/mol
Correct Answer:
Verified
Q64: The reaction C4H10
Q65: An increase in the temperature of the
Q67: The rate law for the reaction H2O2
Q68: The activation energy for the following first-order
Q69: When the concentrations of reactant molecules are
Q72: Given that Ea for a certain biological
Q75: The following reaction in aqueous solution was
Q76: Solids cannot react with gases.
A)1 and 2
B)1
Q92: The activation energy of a certain uncatalyzed
Q98: For the reaction X2 + Y
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents