The gas phase reaction of nitrogen dioxide and carbon monoxide was found by experiment to be second-order with respect to NO2, and zeroth-order with respect to CO below 25°C. NO2 + CO NO + CO2
Which one of the following mechanisms is consistent with the observed reaction order?
A) NO2 + 2CO 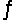 N + 2CO2 fast N + NO2 2NO slow
N + 2CO2 fast N + NO2 2NO slow
B) NO2 + 2CO N + 2CO2 slow N + NO2 2NO fast
C) NO2 + NO2 NO3 + NO fast NO3 + CO NO2 + CO2 slow
D) NO2 + NO2 NO3 + NO slow NO3 + CO NO2 + CO2 fast
Correct Answer:
Verified
Q56: For the chemical reaction A
Q59: The isomerization of methyl isocyanide, CH3NC
Q64: The reaction C4H10
Q65: A reaction mechanism usually is
A)the same as
Q67: The rate law for the reaction H2O2
Q68: The activation energy for the following first-order
Q73: The activation energy for the reaction
Q80: The isomerization of cyclopropane follows first order
Q83: Complete the following statement: A catalyst
A) increases
Q97: Which of the following statements is false
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents