Hydrogen iodide decomposes according to the equation 2HI(g) 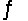 H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of HI at equilibrium.
H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of HI at equilibrium.
A) 0.138 M
B) 0.220 M
C) 0.550 M
D) 0.275 M
E) 0.0275 M
Correct Answer:
Verified
Q20: Which is the correct equilibrium constant expression
Q21: For the following reaction at equilibrium
Q22: For the following reaction at equilibrium,
Q23: Consider the following equilibria: 2SO3(g) 
Q24: For the equilibrium reaction 2SO2(g)+ O2(g)
Q26: At 400ºC, Kc = 64 for the
Q27: For the following reaction at equilibrium,
Q28: For the reaction SO2(g)+ NO2(g) 
Q29: Consider the reaction N2(g)+ O2(g) 
Q30: For the following reaction at equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents