A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively.These ions react according to the following chemical equation 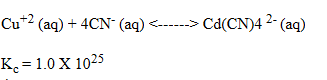
What will be the concentration of CN-(aq)at equilibrium?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: At 700 K, the reaction 2SO2(g)+ O2(g)
Q37: At 700 K, the reaction 2SO2(g)+ O2(g)
Q38: For the reaction PCl3(g)+ Cl2(g) 
Q39: Sodium carbonate, Na2CO3(s), can be prepared by
Q40: At 340 K, Kp = 69 for
Q42: In which of these gas-phase equilibria is
Q43: For the reaction 2NOCl(g) Q44: A quantity of liquid methanol, CH3OH, is Q45: If the reaction 2H2S(g) Q46: The reaction 2SO3(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

