Hydrogen iodide decomposes according to the equation:
2HI(g) 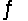
H2(g)+ I2(g), Kc = 0.0156 at 400ºC
A 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of HI at equilibrium.
Correct Answer:
Verified
Q73: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q74: The dissociation of solid silver chloride in
Q75: The data below refer to the following
Q76: Consider the reaction N2(g)+ 3H2(g) 
Q77: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q79: The data below refer to the following
Q80: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q81: Calcium carbonate decomposes at high temperatures to
Q82: Kc for the reaction CO2(g)+ H2(g)
Q83: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents