Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide.
CaCO3(s) 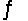
CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C.A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C.Ignoring the volume occupied by the solid, what will be the overall mass percent of carbon in the solid once equilibrium is reached?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q76: Consider the reaction N2(g)+ 3H2(g) 
Q77: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q78: Hydrogen iodide decomposes according to the equation:
2HI(g)
Q79: The data below refer to the following
Q80: Consider the equilibrium equation C(s)+ H2O(g)+ 2296
Q82: Kc for the reaction CO2(g)+ H2(g)
Q83: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q84: Kc for the reaction CO2(g)+ H2(g)
Q85: Kc for the reaction CO2(g)+ H2(g)
Q86: Kc for the reaction CO2(g)+ H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents