The equilibrium between carbon dioxide gas and carbonic acid is very important in biology and environmental science.
CO2(aq)+ H2O(l) 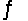
H2CO3(aq)
Based on the equilibrium constant reported for this reaction (Kc = 1.70 * 10-3), there will be more carbon dioxide in solution than carbonic acid.
Correct Answer:
Verified
Q84: Kc for the reaction CO2(g)+ H2(g)
Q85: Kc for the reaction CO2(g)+ H2(g)
Q86: Kc for the reaction CO2(g)+ H2(g)
Q87: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q88: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q90: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q91: What is the correct equilibrium constant expression
Q92: Consider the following equilibrium,
4NH3(g)+ 3O2(g) 
Q93: Two moles of PCl5 are placed in
Q94: The equilibrium constant for the chemical equation
2NO(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents