Multiple Choice
A 0.10 M NH3 solution is 1.3% ionized. Calculate the H+ ion concentration. NH3 + H2O 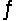 NH4+ + OH-
NH4+ + OH-
A) 1.3 * 10-3 M
B) 7.7 * 10-2 M
C) 7.7 * 10-12 M
D) 0.13 M
E) 0.10 M
Correct Answer:
Verified
Related Questions
Q23: Calculate the pH of a 0.14 M
Q24: What is the pH of 10.0 mL
Q27: What is the OH- ion concentration in
Q34: Calculate the pH of a 0.10 M
Q35: A 0.14 M HNO2 solution is 5.7%
Q40: Calculate the pH of 2.6 *10-2 M
Q49: What is the pH of a 0.014
Q51: What is the pH of a 0.001
Q57: Calculate the pH of a 1.6 M
Q59: Calculate the hydrogen ion concentration in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents