Predict the direction in which the equilibrium will lie for the reaction H2CO3 + F-
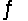 HCO3- + HF. Ka1( H2CO3) = 4.2 * 10-7; Ka(HF) = 7.1 * 10-4
HCO3- + HF. Ka1( H2CO3) = 4.2 * 10-7; Ka(HF) = 7.1 * 10-4
A) to the right
B) to the left
C) in the middle
Correct Answer:
Verified
Q64: Arrange the acids HOCl, HClO3, and HClO2
Q73: When comparing acid strength of binary acids
Q75: In the reaction CaO(s)+ SO2(g) 
Q76: Predict the direction in which the equilibrium
Q79: Which of these acids is the strongest?
A)H2SO3
B)H2SeO3
C)H2TeO3
Q81: An aqueous solution of KCl would be
A)neutral
B)basic
C)acidic
Q89: Which of these species will act as
Q96: Calculate the concentration of chromate ion (CrO42-)
Q103: What is the pH of a 0.20
Q107: Which one of these salts will form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents