Consider the weak bases below and their Kb values: 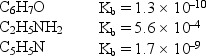 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
A) C5H5NH+ < C6H7OH < C2H5NH
B) C6H7OH < C5H5NH+ < C2H5NH
C) C5H5NH+ < C2H5NH3+ < C6H7OH
D) C6H7OH < C2H5NH3+< C5H5NH+
E) C2H5NH3+< C5H5NH+ < C6H7OH
Correct Answer:
Verified
Q89: In 0.10 M KCN, the chemical species
Q91: For H3PO4, Ka1 = 7.3 * 10-3,
Q92: Which response gives the products of hydrolysis
Q94: Hydrosulfuric acid is a diprotic acid, for
Q106: Which one of these salts will form
Q109: Which one of these salts will form
Q110: Calculate the pH of a 0.021 M
Q112: For H3PO4, Ka1 = 7.3 * 10-3,
Q114: Which one of these salts will form
Q126: Which one of these salts will form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents